Short Answer
Consider the reaction CO(g)+ 2H2(g) 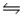
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate value of the equilibrium constant (Kp)for this reaction at 25°C.
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Calculate the free energy of formation
Q46: In the gas phase, methyl isocyanate
Q47: For any pure substance, if
Q48: What is the free energy change
Q49: Which response includes all of the following
Q51: Using the thermodynamic data provided below, calculate
Q52: Choose the substance with the higher entropy
Q53: The entropy of a perfectly ordered crystalline
Q54: Choose the substance with the higher entropy
Q122: Which response includes all of the