Essay
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 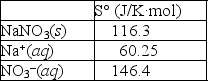 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Correct Answer:

Verified
90.4 J/K·mol; solubi...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q54: Which response includes all the following
Q56: With respect to the system only,
Q58: Choose the substance with the higher entropy
Q59: Arrange the following substances in the
Q60: Which of the following processes would
Q61: Rubidium has a heat of vaporization
Q66: Aluminum forms a layer of aluminum
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q68: The normal boiling point of acetic
Q99: Which of the following is expected to