Multiple Choice
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from further corrosion. 4Al(s) + 3O2(g) 2Al2O3(s)
Using the thermodynamic data provided below, calculate S° for this reaction. 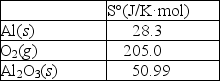
A) 182.3 J/K·mol
B) 131.5 J/K·mol
C) -182.3 J/K·mol
D) -626.2 J/K·mol
E) -802.9 J/K·mol
Correct Answer:

Verified
Correct Answer:
Verified
Q54: Which response includes all the following
Q56: With respect to the system only,
Q61: Rubidium has a heat of vaporization
Q63: Using the thermodynamic data provided below, calculate
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q68: The normal boiling point of acetic
Q69: The normal melting point sulfur is
Q70: The free energy of formation of nitric
Q71: Using the thermodynamic data provided below, calculate
Q99: Which of the following is expected to