Short Answer
For the reaction SbCl5(g) 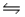
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Dissolving an ionic solid in water always
Q78: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q79: Which of the following is consistent
Q81: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q82: Which one of the following reactions
Q84: Which of these species has the highest
Q85: At 700 K, the equilibrium constant
Q86: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q87: Which of the following is consistent
Q88: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span