Short Answer
For the reaction CuS(s)+ H2(g) 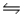
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Dissolving an ionic solid in water always
Q76: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q77: Which of these species would you expect
Q78: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q79: Which of the following is consistent
Q82: Which one of the following reactions
Q83: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q84: Which of these species has the highest
Q85: At 700 K, the equilibrium constant
Q86: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"