Short Answer
The free energy of formation of nitric oxide, NO, at 1000 K (roughly the temperature in an automobile engine during ignition)is about 78 kJ/mol.Calculate the equilibrium constant Kp for the reaction N2(g)+ O2(g) 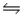
2NO(g)at this temperature.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q56: With respect to the system only,
Q56: With respect to the system only,
Q66: Aluminum forms a layer of aluminum
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q68: The normal boiling point of acetic
Q69: The normal melting point sulfur is
Q71: Using the thermodynamic data provided below, calculate
Q72: HI has a normal boiling point
Q73: The entropy change <span class="ql-formula"
Q74: Sulfur can be separated from lead