Short Answer
Consider the reaction CO(g)+ 2H2(g) 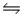
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate S°(H2(g)).
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Dissolving an ionic solid in water always
Q56: With respect to the system only,
Q71: Using the thermodynamic data provided below, calculate
Q72: HI has a normal boiling point
Q73: The entropy change <span class="ql-formula"
Q74: Sulfur can be separated from lead
Q77: Which of these species would you expect
Q78: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q79: Which of the following is consistent
Q81: For the reaction CuS(s)+ H<sub>2</sub>(g) <img