Multiple Choice
Given the following standard reduction potentials, 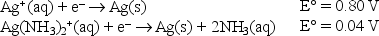 calculate the formation constant of Ag(NH3) 2+ at 25°C.
calculate the formation constant of Ag(NH3) 2+ at 25°C.
A) 6.1 × 10-15
B) 1.5 × 10-13
C) 6.9 × 1012
D) 1.6 × 1014
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: Aluminum does not corrode as does iron,
Q46: Calculate the cell emf for the
Q48: Which of these metals will not be
Q49: Calculate E°<sub>cell</sub> for the following electrochemical cell:<br>Ni(s)|
Q51: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q52: Consider an electrochemical cell involving the
Q54: The measured voltage of the cell Pt(s)|
Q55: Write the formula of the strongest reducing
Q117: Consider the following reaction: 2Fe<sup>2+</sup>(aq) +
Q150: Complete and balance the following redox