Short Answer
Write the formula of the strongest reducing agent.given the following standard reduction potentials in acid solution 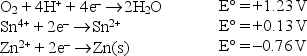
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Using a table of standard electrode potentials,
Q50: Given the following standard reduction potentials, <img
Q51: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q52: Consider an electrochemical cell involving the
Q54: The measured voltage of the cell Pt(s)|
Q54: Which of the following is true concerning
Q56: A current of 2.50 A was passed
Q60: Consider an electrochemical cell based on the
Q117: Consider the following reaction: 2Fe<sup>2+</sup>(aq) +
Q153: For the electrochemical cell Pt(s) | H<sub>2</sub>(1