Short Answer
A gas is allowed to expand, at constant temperature, from a volume of 1.0 L to 10.1 L against an external pressure of 0.50 atm. If the gas absorbs 250 J of heat from the surroundings, what are the values of q, w, and E? 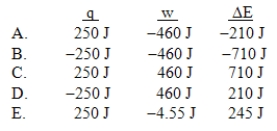
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Calculate the enthalpy of reaction for
Q14: According to the first law of thermodynamics:<br>A)
Q22: Find <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q28: Which of the following processes is
Q43: The residential rate for natural gas is
Q68: Copper metal has a specific heat of
Q69: What is the standard enthalpy of formation
Q71: Methanol (CH<sub>3</sub>OH)burns according to the equation
Q73: Ethanol undergoes combustion in oxygen to
Q74: Find the standard enthalpy of formation