Multiple Choice
Based on the phase diagram shown below, how will the melting point of the substance change if the pressure is increased above 1 atm? 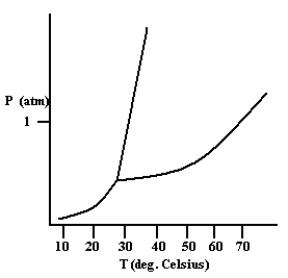
A) The melting point will decrease.
B) The melting point will remain the same.
C) The melting point will increase.
D) The substance will not melt at pressures of 1 atm and above; instead, the solid sublimes to form the gas phase.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Given the following compound and its boiling
Q3: What phase exists at the point labeled
Q4: The molecular property related to the ease
Q5: How much enthalpy is necessary to heat
Q11: Of the pair of compounds given, which
Q12: Given that the heat of vaporization of
Q29: Identify the dominant (strongest)type of intermolecular force
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q113: Indicate all the types of intermolecular forces
Q119: The number of atoms in a body-centered