Short Answer
How much enthalpy is necessary to heat 10.0 g of solid benzene (C6H6)at 0.0°C to benzene vapor at 100°C? 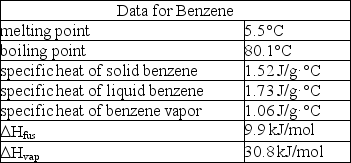
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Use the graph of vapor pressure to
Q2: Given the following compound and its boiling
Q3: What phase exists at the point labeled
Q4: The molecular property related to the ease
Q7: Based on the phase diagram shown below,
Q11: Of the pair of compounds given, which
Q12: Given that the heat of vaporization of
Q29: Identify the dominant (strongest)type of intermolecular force
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q113: Indicate all the types of intermolecular forces