Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3- 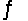 H2PO4- + HNO3 Ka(H3PO4) = 7.5 * 10-3
H2PO4- + HNO3 Ka(H3PO4) = 7.5 * 10-3
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Acid strength decreases in the series: HCl
Q7: The pH of a 0.6 M solution
Q10: Write the chemical formula for perchloric acid.
Q12: The equilibrium constant for the reaction C<sub>7</sub>H<sub>15</sub>COOH(aq)+
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q35: The pH of a Ba(OH)<sub>2</sub> solution is
Q58: Arrange the acids HOBr, HBrO<sub>3</sub>, and HBrO<sub>2</sub>
Q82: Calculate the concentration of chromate ion (CrO<sub>4</sub><sup>2-</sup>)
Q87: When comparing acid strength of binary acids
Q134: What is the H<sup>+</sup> ion concentration in