Multiple Choice
The equilibrium constant for the reaction C7H15COOH(aq) + HCOO-(aq) 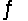 C7H15COO-(aq) + HCOOH(aq)
C7H15COO-(aq) + HCOOH(aq)
Is 7.23 * 10-2 at 25°C. If Ka for formic acid (HCOOH) is 1.77 * 10-4, what is the acid dissociation constant for C7H15COOH?
A) 2.45 * 10-3
B) 4.08 * 10-2
C) 7.81 * 10-4
D) 1.00 * 10-4
E) 1.28 * 10-5
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Calculate the concentration of malonate ion (C<sub>3</sub>H<sub>2</sub>O<sub>4</sub><sup>2-</sup>)
Q7: The pH of a 0.6 M solution
Q8: Predict the direction in which the equilibrium
Q10: Write the chemical formula for perchloric acid.
Q13: In the reaction HNO<sub>3</sub> + NH<sub>3</sub> <img
Q14: Predict the direction in which the equilibrium
Q15: An unknown substance was added to a
Q53: The OH<sup>-</sup> concentration in a 2.5 *
Q58: Arrange the acids HOBr, HBrO<sub>3</sub>, and HBrO<sub>2</sub>
Q87: When comparing acid strength of binary acids