Multiple Choice
Calculate S° at 25°C for the reduction of PbO(s) , 2PbO(s) + C(s) 2Pb(s) + CO2(g) given these absolute entropies: 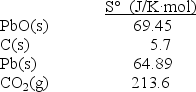
A) +198.8 J/K·mol
B) +488.0 J/K·mol
C) +353.6 J/K·mol
D) -203.3 J/K·mol
E) +203.3 J/K·mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q52: Choose the substance with the higher entropy
Q54: Which response includes all the following
Q58: Choose the substance with the higher entropy
Q99: Sulfur can be separated from lead
Q101: Consider the gas phase reaction shown
Q103: The equilibrium constant for the reaction
Q104: The equilibrium constant at 427°C for
Q105: Find the temperature at which K<sub>p</sub>
Q128: Which one of the following reactions