Multiple Choice
The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) 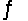 2NH3(g) is Kp = 9.4 10-5. Calculate the value of G° for the reaction under these conditions.
2NH3(g) is Kp = 9.4 10-5. Calculate the value of G° for the reaction under these conditions.
A) -33 kJ/mol
B) -54 kJ/mol
C) 54 kJ/mol
D) 33 kJ/mol
E) 1.3 J/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q58: Choose the substance with the higher entropy
Q99: Sulfur can be separated from lead
Q101: Consider the gas phase reaction shown
Q102: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q103: The equilibrium constant for the reaction
Q105: Find the temperature at which K<sub>p</sub>
Q108: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q109: Using the thermodynamic data provided below, calculate
Q128: Which one of the following reactions