Multiple Choice
Calculate G° for the reaction 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) . 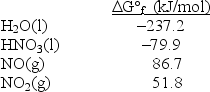
A) 8.7 kJ/mol
B) 192 kJ/mol
C) -414 kJ/mol
D) -192 kJ/mol
E) -155 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: What is the free energy change
Q72: HI has a normal boiling point
Q77: Determine the equilibrium constant K<sub>p</sub> at
Q79: How does the entropy change when a
Q80: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img
Q82: Which species will have the greatest absolute
Q82: Which one of the following reactions
Q83: Calculate the equilibrium constant for the
Q84: How does the entropy change when a
Q103: The following reaction is spontaneous under