Short Answer
For the reaction 3H2(g)+ N2(g) 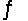 2NH3(g), Kc = 9.0 at 350°C. Calculate G° at 350°C.
2NH3(g), Kc = 9.0 at 350°C. Calculate G° at 350°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q48: What is the free energy change
Q72: HI has a normal boiling point
Q75: K<sub>w</sub> for the auto-ionization of water,
Q77: Determine the equilibrium constant K<sub>p</sub> at
Q79: How does the entropy change when a
Q81: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q82: Which species will have the greatest absolute
Q83: Calculate the equilibrium constant for the
Q84: How does the entropy change when a
Q103: The following reaction is spontaneous under