Multiple Choice
In the gas phase, formic acid forms a dimmer, 2HCOOH(g) 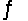 (HCOOH) 2(g) . For this reaction, H° = -60.1 kJ/mol and G° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (Kp) for this reaction at 75 °C.
(HCOOH) 2(g) . For this reaction, H° = -60.1 kJ/mol and G° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (Kp) for this reaction at 75 °C.
A) 8960
B) 273
C) 0.120
D) 8.33
E) 1.12 10-4
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Assuming <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q17: For the reaction 2C(graphite)+ H<sub>2</sub>(g) <span
Q54: Which response includes all the following
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q84: Which of these species has the highest
Q88: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q89: At 1500°C the equilibrium constant for
Q94: The standard free energy of formation of
Q95: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q96: Using the thermodynamic data provided below, calculate