Multiple Choice
Identify the acid or base behavior of each substance in these reactions: 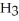
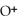 +
+ 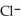 ⇌
⇌ 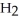 O + HCl
O + HCl
A) 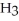
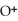 acts as an acid,
acts as an acid,  acts as a base,
acts as a base,  O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
B) 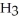
 acts as an base,
acts as an base,  acts as a acid,
acts as a acid, 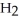 O acts an acid, HCl acts as a base.
O acts an acid, HCl acts as a base.
C) 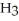
 acts as an acid,
acts as an acid, 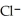 acts as a base,
acts as a base, 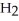 O acts an base, HCl acts as a acid.
O acts an base, HCl acts as a acid.
D) 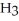
 acts as an base,
acts as an base,  acts as a acid,
acts as a acid,  O acts an base, HCl acts as an acid.
O acts an base, HCl acts as an acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: According to the following reaction,which molecule is
Q51: If the pH of a solution was
Q71: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q73: Which of the following reactions illustrates an
Q78: Suggest why people once washed their hands
Q128: Qualitatively, what happens to the hydronium ion
Q129: In the following buffer system, what happens
Q133: Which of the above images would best
Q135: Acetic acid, shown below, has 4 hydrogen
Q146: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the