Multiple Choice
Acetic acid, shown below, has 4 hydrogen atoms-one bonded to an oxygen and three bonded to a carbon. When this molecule behaves as an acid, it donates only the hydrogen bonded to the oxygen. The hydrogens bonded to the carbon remain intact. Why? 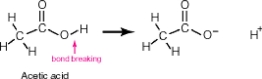
A) The oxygen is much better at accommodating a negative charge than is carbon.
B) The hydrogen attached to the oxygen extends farther away from the center of the molecule.
C) The carbon is bonded to three hydrogens while the oxygen is bonded to only one hydrogen.
D) The oxygen within acetic acid has two lone pairs of electrons that help to destabilize the oxygen-hydrogen bond.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: According to the following reaction,which molecule is
Q51: If the pH of a solution was
Q71: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q73: Which of the following reactions illustrates an
Q78: Suggest why people once washed their hands
Q128: Qualitatively, what happens to the hydronium ion
Q129: In the following buffer system, what happens
Q131: Identify the acid or base behavior of
Q133: Which of the above images would best
Q146: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6597/.jpg" alt=" -Which of the