Multiple Choice
The main component of bleach is sodium hypochlorite, NaOCl, which consists of sodium ions, Na⁺, and hypochlorite ions, 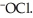 What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
What products are formed when this compound is reacted with the hydrochloric acid, HCl, of toilet bowl cleaner?
A) The products are NaCl, 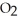 , and
, and 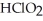 .
.
B) The products are NaOH, 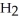 O, and
O, and 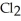
C) The products are NaCl and HOCl.
D) The products are NaOH, 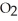 , and
, and 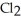
Correct Answer:

Verified
Correct Answer:
Verified
Q74: What best describes what happens when an
Q109: Sodium bicarbonate, NaHC <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="Sodium bicarbonate,
Q110: Hydrogen chloride is added to a buffer
Q112: In behaving as both an acid and
Q114: How does a base like NH<sub>3</sub> raise
Q116: Does the hydride ion, H⁻, tend to
Q117: Along with the pH scale, there is
Q126: What happens to the pH of an
Q139: If you had a 1 M solution
Q140: For the following reaction,identify whether the compound