Multiple Choice
Does the hydride ion, H⁻, tend to behave as an acid or a base?
A) Hydride ion is unstable and re-reacts with water to form an acid in the form of the hydronium ion, H3O⁺.
B) Hydride ion has a pair of electrons and acts like a Lewis base since it has a strong tendency to lend or donate that electron pair.
C) Although the hydride ion has two electrons, they do not pair together. Thus, the hydride ion is incapable of acting as a Lewis base and therefore tends to behave as an acid.
D) Hydride ion's makeup consists of one proton and two electrons. As such, its tendency is to form neutral hydrogen gas, 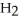 , and therefore is neither acidic nor basic, but neutral.
, and therefore is neither acidic nor basic, but neutral.
Correct Answer:

Verified
Correct Answer:
Verified
Q74: What best describes what happens when an
Q112: In behaving as both an acid and
Q113: The main component of bleach is sodium
Q114: How does a base like NH<sub>3</sub> raise
Q117: Along with the pH scale, there is
Q120: What happens to the pH of a
Q121: In the following buffer system, what happens
Q126: What happens to the pH of an
Q139: If you had a 1 M solution
Q170: According to the following reaction,which molecule is