Multiple Choice
Some molecules are able to stabilize a negative charge by passing it from one atom to the next by a flip-flopping of double bonds. This occurs when the negative charge is one atom away from an oxygen double bond as follows. Note that the curved arrows indicate the movement of electrons: 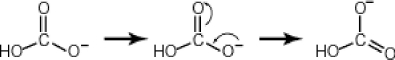 Why then is sulfuric acid so much stronger an acid than carbonic acid?
Why then is sulfuric acid so much stronger an acid than carbonic acid? 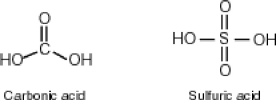
A) In sulfuric acid, the negative charge is able to flip-flop to two additional oxygens rather than only one as is the case for carbonic acid.
B) The two double bonded oxygens in 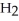
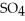 tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
tend to destabilize the single bonded oxygens once the hydrogen ions form, thus making sulfuric acid more acidic.
C) Since carbonic acid has resonance stabilization and sulfuric acid does not, sulfuric acid is less stable and more acidic.
D) The acid strength of two comparative molecules is directly proportional to the number of number of oxygens directly bonded to the central atom. Sulfuric acid, having four such oxygens, is more acidic.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Sodium hydroxide, NaOH, is a very strong
Q37: Which of the following statements about buffers
Q39: According to the following reaction,which molecule is
Q42: Which should be a stronger base: ammonia,
Q116: For the following acid-base reaction,identify what compound
Q122: Which of the following statements describes a
Q140: For the following reaction,identify whether the compound
Q154: Pour vinegar onto beach sand from the
Q161: According to the following reaction,which molecule is
Q174: Why might an area with a large