Multiple Choice
What is the hydroxide ion concentration in an aqueous solution when the hydronium ion concentration equals 1 × 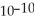 moles per liter?
moles per liter?
A) The concentration of hydroxide ions is 1 × 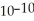 moles per liter.
moles per liter.
B) The concentration of hydroxide ions is 1 × 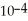 moles per liter.
moles per liter.
C) The concentration of hydroxide ions is 1 × 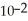 moles per liter.
moles per liter.
D) The concentration of hydroxide ions is 1 × 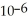 moles per liter.
moles per liter.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: What is a base?<br>A)anything that accepts a
Q57: Which of the following solutions is the
Q73: How readily an acid donates a hydrogen
Q74: Which of the following compounds could never
Q76: What would the concentration of OH<sup>-</sup> be
Q78: What is the hydroxide ion concentration in
Q79: Which of the following items would you
Q80: If you had a 1 M solution
Q121: An acid and a base react to
Q140: For the following reaction,identify whether the compound