Multiple Choice
What is the hydroxide ion concentration in an aqueous solution where the pH = 5?
A) The hydronium ion concentration equals 1 × 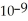 .
.
B) The hydronium ion concentration equals 1 × 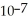 .
.
C) The hydronium ion concentration equals 1 × 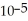 .
.
D) The hydronium ion concentration equals 1 × 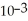 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: What is the relationship between the hydroxide
Q57: Which of the following solutions is the
Q73: How readily an acid donates a hydrogen
Q74: Which of the following compounds could never
Q75: What is the hydroxide ion concentration in
Q76: What would the concentration of OH<sup>-</sup> be
Q79: Which of the following items would you
Q80: If you had a 1 M solution
Q83: If you had a 1 M solution
Q150: What happens to the corrosive properties of