Multiple Choice
Sodium hydroxide is added to a buffer solution of ammonia, N 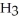 , and ammonium chloride,
, and ammonium chloride, 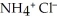 . What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
. What is the effect on the concentration of ammonia? What is the effect on the concentration of ammonium chloride?
A) Sodium hydroxide reacts with both the ammonia and ammonium chloride, so the concentration of both decreases.
B) Sodium hydroxide reacts with only the ammonium chloride, so the concentration of the ammonium chloride decreases and the concentration of ammonia increases.
C) Sodium hydroxide reacts with both the ammonia and ammonium chloride, so the concentration of both increases.
D) Sodium hydroxide reacts with only the ammonia, so the concentration of the ammonium chloride increases and the concentration of ammonia decreases.
Correct Answer:

Verified
Correct Answer:
Verified
Q21: What happens to the pH of soda
Q42: Which should be a stronger base: ammonia,
Q46: Why is the H-F bond so much
Q49: The overall rate at which humans are
Q50: In the following buffer system, what happens
Q90: What is an acid?<br>A)anything that donates hydrogen
Q122: Which of the following statements describes a
Q140: For the following reaction,identify whether the compound
Q154: Pour vinegar onto beach sand from the
Q161: According to the following reaction,which molecule is