Multiple Choice
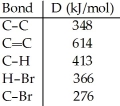
Using the table of bond dissociation energies, the ΔH for the following gas-phase reaction is ________ kJ. 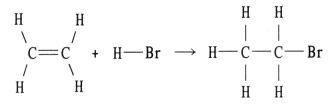
A) 291
B) 2017
C) -57
D) -356
E) -291
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Of the bonds C-C, C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2701/.jpg"
Q8: The Lewis structure of HCN (H bonded
Q11: The strength of a covalent bond is
Q12: Ni<sup>2+</sup> ions are represented by the electron
Q56: The Lewis structure of AsH<sub>3</sub> shows _
Q84: Atoms surrounded by eight valence electrons tend
Q89: In ionic bond formation,the lattice energy of
Q108: Which of the following does not have
Q125: There are _ valence electrons in the
Q143: Based on the octet rule,magnesium most likely