Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 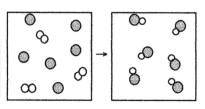
A) A + B → AB
B) A + 3B → 3AB
C) A2 + B → AB
D) A2 + 2B → 2AB
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q123: Strontium phosphate reacts with sulfuric acid to
Q124: Oxygen can be produced from the catalytic
Q125: How many grams of calcium chloride are
Q126: How many moles are in 7.8 g
Q127: The balanced equation for the decomposition of
Q129: How many anions are there in 4.50
Q130: Which one of the following is not
Q131: Identify one mole of a substance.<br>A)32.0 grams
Q132: The following diagram represents the reaction of
Q133: How many cations are in 0.500 g