Multiple Choice
The following diagram represents the reaction of A (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 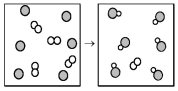
A) A2 + 2B → 2AB;A2 is the limiting reactant.
B) A2 + 2B → 2AB;B is the limiting reactant.
C) 4A2 + 6B → 6AB;A2 is the limiting reactant.
D) 4A2 + 6B → 6AB;B is the limiting reactant.
Correct Answer:

Verified
Correct Answer:
Verified
Q127: The balanced equation for the decomposition of
Q128: What is the balanced chemical equation for
Q129: How many anions are there in 4.50
Q130: Which one of the following is not
Q131: Identify one mole of a substance.<br>A)32.0 grams
Q133: How many cations are in 0.500 g
Q134: 5.0 g of nitrogen is reacted with
Q135: How many oxygen atoms are there in
Q136: When carbon dioxide dissolves in water,H<sup>+</sup> is
Q137: Isoeugenol is the compound which gives the