Multiple Choice
Three different substances,A2X,A2Y,and A2Z,were dissolved in water with the following results.(Water molecules are omitted for clarity. ) Which of the substances is the strongest electrolyte,and which is the weakest? 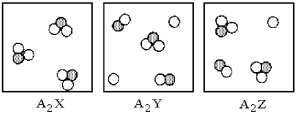
A) A2X is the strongest electrolyte and A2Y is the weakest electrolyte.
B) A2Y is the strongest electrolyte and A2X is the weakest electrolyte.
C) A2Y is the strongest electrolyte and A2Z is the weakest electrolyte.
D) A2Z is the strongest electrolyte and A2Y is the weakest electrolyte.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: The number of milliliters of 0.250 M
Q17: What ion is provided when Arrhenius bases
Q18: A hydrocarbon of unknown formula C<sub>x</sub>H<sub>y</sub> was
Q19: Metals that do not dissolve in non-oxidizing
Q20: Write a net ionic equation for the
Q22: Assume that an aqueous solution of hydroxide
Q23: What is the molarity of a solution
Q24: The balanced net ionic equation for the
Q25: In the reaction 2 H<sub>2</sub>O<sub>2</sub>(l)→ 2 H<sub>2</sub>O(l)+
Q26: What is the oxidation number change for