Multiple Choice
Assume that an aqueous solution of hydroxide ion,OH-,represented by unshaded spheres,is allowed to mix with a solution of an acid,HnA,represented by gray spheres.Three possible outcomes are represented by boxes (a) -(c) ,where the black spheres represent An-,the anion of the acid.For clarity H2O molecules are not shown. 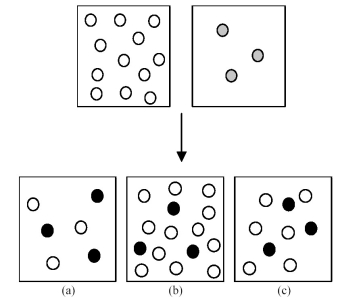
-Which outcome corresponds to the reaction: H2SO4 + 2 OH- → 2 H2O + SO42-?
A) box (a)
B) box (b)
C) box (c)
D) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q17: What ion is provided when Arrhenius bases
Q18: A hydrocarbon of unknown formula C<sub>x</sub>H<sub>y</sub> was
Q19: Metals that do not dissolve in non-oxidizing
Q20: Write a net ionic equation for the
Q21: Three different substances,A<sub>2</sub>X,A<sub>2</sub>Y,and A<sub>2</sub>Z,were dissolved in water
Q23: What is the molarity of a solution
Q24: The balanced net ionic equation for the
Q25: In the reaction 2 H<sub>2</sub>O<sub>2</sub>(l)→ 2 H<sub>2</sub>O(l)+
Q26: What is the oxidation number change for
Q27: What reagent would distinguish between Ba<sup>2+</sup> and