Multiple Choice
Assume that an aqueous solution of hydroxide ion,OH-,represented by unshaded spheres,is allowed to mix with a solution of an acid,HnA,represented by gray spheres.Three possible outcomes are represented by boxes (a) -(c) ,where the black spheres represent An-,the anion of the acid.For clarity H2O molecules are not shown. 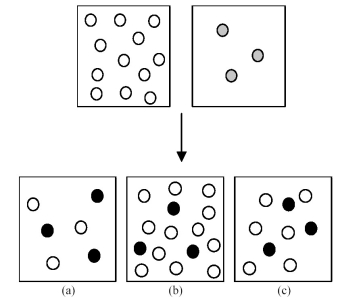
-Which outcome corresponds to the reaction: HCN + OH- → H2O + CN-?
A) box (a)
B) box (b)
C) box (c)
D) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Q4: What reagent would distinguish between Ag<sup>+</sup> and
Q5: The oxidation state of chlorine in ClO<sub>4</sub><sup>-</sup>
Q6: Based on the positions in the periodic
Q7: What is the oxidation number of the
Q8: When 125 mL of 0.500 M AgNO<sub>3</sub>
Q10: In an acid-base neutralization reaction 43.74 mL
Q11: If the reaction of phosphate ion with
Q12: The unknown acid H<sub>2</sub>X can be neutralized
Q13: Write a balanced net ionic equation for
Q14: Which one of the following compounds is