Multiple Choice
Based on the positions in the periodic table of elements A,B,and C,which of the following reactions would you expect to occur? 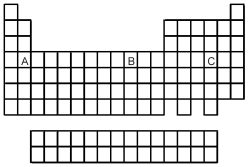
A) A2+ + B → A + B2+
B) B2+ + C → B + C2+
C) C + A → C2- + A2+
D) None of the reactions would be expected to occur.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Molarity is defined as<br>A)moles of solute per
Q2: What is the oxidation number of the
Q3: In a solution prepared by mixing CH<sub>3</sub>OH
Q4: What reagent would distinguish between Ag<sup>+</sup> and
Q5: The oxidation state of chlorine in ClO<sub>4</sub><sup>-</sup>
Q7: What is the oxidation number of the
Q8: When 125 mL of 0.500 M AgNO<sub>3</sub>
Q9: Assume that an aqueous solution of hydroxide
Q10: In an acid-base neutralization reaction 43.74 mL
Q11: If the reaction of phosphate ion with