Multiple Choice
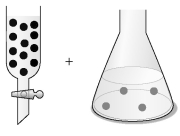
-The concentration of an aqueous solution of Fe2+ can be determined by a redox titration with aqueous bromate ion,BrO3⁻: 6 Fe2+ (aq) + BrO3⁻ (aq) + 6 H⁺ (aq) → 6 Fe3+ (aq) + Br⁻ (aq) + 3 H2O (l)
Assume that the black spheres in the buret represent BrO3⁻ ions,the gray spheres in the flask represent Fe2+ ions,the concentration of the BrO3⁻ ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the Fe2+ in the flask,and what fraction of the BrO3⁻ solution in the buret must be added to the flask to react with all the Fe2+ ions?
A) 0.0200 M Fe2+;1/18 of the BrO3⁻ must be added.
B) 0.0200 M Fe2+;1/3 of the BrO3⁻ must be added.
C) 0.0400 M Fe2+;1/18 of the BrO3⁻ must be added.
D) 0.0400 M Fe2+;1/3 of the BrO3⁻ must be added.
Correct Answer:

Verified
Correct Answer:
Verified
Q178: Glucose,C<sub>6</sub>H<sub>1</sub><sub>2</sub>0<sub>6</sub>,can be represented by the molecular model
Q179: Using the following portion of the activity
Q180: The oxidation number of hydrogen in CaH<sub>2</sub>
Q181: What is the concentration of FeBr<sub>3</sub> in
Q182: The oxidation number of chromium in Ag<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub>
Q184: The chemical formula for nitric acid is<br>A)HNO<sub>2</sub>(aq).<br>B)HNO<sub>3</sub>(aq).<br>C)H<sub>2</sub>NO<sub>3</sub>(aq).<br>D)H<sub>2</sub>NO<sub>2</sub>(aq).
Q185: The number of grams of NaCl required
Q186: What is the oxidation number change for
Q187: Which of the following compounds is not
Q188: What is the concentration of NO<sub>3</sub><sup>-</sup> ions