Multiple Choice
Glucose,C6H1206,can be represented by the molecular model shown below.If 1.00 mol of glucose is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 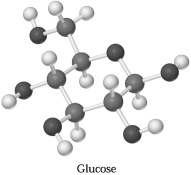
A) 1.00 mol CO2 and 2.00 mol H2O
B) 6.00 mol CO2 and 6.00 mol H2O
C) 6.00 mol CO2 and 12.0 mol H2O
D) 12.0 mol CO2 and 12.0 mol H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q173: How many milliliters of a 6.0 M
Q174: What is the oxidation number change for
Q175: Which one of the following compounds behaves
Q176: What is the oxidation number of the
Q177: Which one of the following compounds is
Q179: Using the following portion of the activity
Q180: The oxidation number of hydrogen in CaH<sub>2</sub>
Q181: What is the concentration of FeBr<sub>3</sub> in
Q182: The oxidation number of chromium in Ag<sub>2</sub>Cr<sub>2</sub>O<sub>7</sub>
Q183: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -The concentration of