Multiple Choice
Assume that the unshaded spheres in the buret represent H+ ions,the shaded spheres in the flask represent OH- ions,and you are carrying out a titration of the base with the acid. 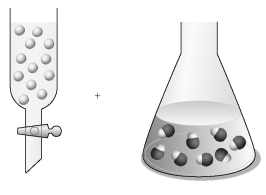
-If the volumes in the buret and the flask are identical and the concentration of the acid in the buret is 0.500 M,what is the concentration of the base in the flask?
A) 0.333 M
B) 0.500 M
C) 0.667 M
D) 0.750 M
Correct Answer:

Verified
Correct Answer:
Verified
Q124: In the following reaction,the reducing agent is
Q125: Which outcome corresponds to the combination of
Q126: What is the oxidation number of the
Q127: Which species functions as the reducing agent
Q128: Assume that the conductivity of a solution
Q130: What is the oxidation number of the
Q131: Which outcome corresponds to the combination of
Q132: Which pair of compounds is soluble in
Q133: If the reaction of phosphate ion with
Q134: A hydrocarbon of unknown formula C<sub>x</sub>H<sub>y </sub>was