Multiple Choice
Which substance in each of the following pairs is expected to have the larger dispersion forces? 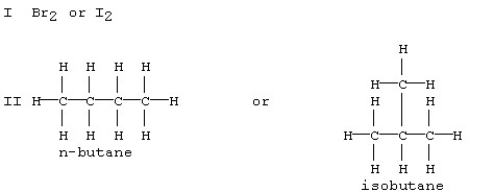
A) Br2 in set I and n-butane in set II
B) Br2 in set I and isobutane in set II
C) I2 in set I and n-butane in set II
D) I2 in set I and isobutane in set II
Correct Answer:

Verified
Correct Answer:
Verified
Q191: Which molecule has a central atom that
Q192: Which drawing below best represents hydrogen bonding
Q193: Which drawing best accounts for the polarity
Q194: What is the geometry around the central
Q195: What is the hybridization of the oxygen
Q197: The MO diagram below is appropriate for
Q198: What is the bond angle in the
Q199: The VSEPR model predicts the H-B-H bond
Q200: The C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt="The C
Q201: Which of the following is not a