Multiple Choice
Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 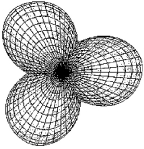
A) H2S
B) CS2
C) NO2-
D) IF2-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q186: Which of the following compounds exhibits hydrogen
Q187: What is the geometry around the central
Q188: A molecule with the formula XF<sub>3</sub> where
Q189: Which of the following is not true?<br>A)The
Q190: Which of the following compounds exhibits only
Q192: Which drawing below best represents hydrogen bonding
Q193: Which drawing best accounts for the polarity
Q194: What is the geometry around the central
Q195: What is the hybridization of the oxygen
Q196: Which substance in each of the following