Multiple Choice
The following drawing is a representation of a reaction for which ΔH° = -22 kJ.This reaction is likely to be 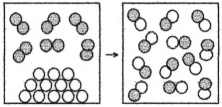
A) nonspontaneous at all temperatures.
B) nonspontaneous at low temperatures and spontaneous at high temperatures.
C) spontaneous at low temperatures and nonspontaneous at high temperatures.
D) spontaneous at all temperatures.
Correct Answer:

Verified
Correct Answer:
Verified
Q126: Which of the following can be interpreted
Q127: Determine the sign of ΔS° for each
Q128: Heat transfer measured in a coffee-cup calorimeter
Q129: For the conversion of ice to water
Q130: The first law of thermodynamics<br>A)defines water energy.<br>B)defines
Q132: Which equation represents the reaction whose ΔH,represents
Q133: The following drawing is a representation of
Q134: At 25°C the heat of fusion of
Q135: Because the number of moles of gas
Q136: Find ΔH° for the reaction C<sub>3</sub>H<sub>8</sub>(g)+ 5