Multiple Choice
The following drawing is a representation of the exothermic reaction in which ozone forms dioxygen. 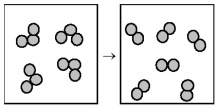
-What are the signs of ΔH and ΔS for this reaction?
A) ΔH = +,ΔS = +
B) ΔH = +,ΔS = -
C) ΔH = -,ΔS = +
D) ΔH = -,ΔS = -
Correct Answer:

Verified
Correct Answer:
Verified
Q128: Heat transfer measured in a coffee-cup calorimeter
Q129: For the conversion of ice to water
Q130: The first law of thermodynamics<br>A)defines water energy.<br>B)defines
Q131: The following drawing is a representation of
Q132: Which equation represents the reaction whose ΔH,represents
Q134: At 25°C the heat of fusion of
Q135: Because the number of moles of gas
Q136: Find ΔH° for the reaction C<sub>3</sub>H<sub>8</sub>(g)+ 5
Q137: Imagine a reaction that results in a
Q138: Calculate the total quantity of heat required