Multiple Choice
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) from 45.0°C to gaseous CCl4 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 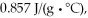 its heat of fusion is
its heat of fusion is 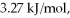 and its heat of vaporization is
and its heat of vaporization is 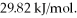
A) 0.681 kJ
B) 1.21 kJ
C) 5.53 kJ
D) 6.06 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q133: The following drawing is a representation of
Q134: At 25°C the heat of fusion of
Q135: Because the number of moles of gas
Q136: Find ΔH° for the reaction C<sub>3</sub>H<sub>8</sub>(g)+ 5
Q137: Imagine a reaction that results in a
Q139: The sign of ΔS° for reaction below
Q140: The heat of combustion per mole for
Q141: When the reaction below is performed in
Q142: Is thermal energy a form of kinetic
Q143: A reaction that absorbs 49.6 kJ from