Multiple Choice
You are given two flasks of equal volume.One contains H2 at 0°C and 1 atm while the other contains CO2 at 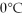 and 2 atm.Which of the following quantities will be the same for both flasks?
and 2 atm.Which of the following quantities will be the same for both flasks?
A) average molecular kinetic energy
B) average molecular speed
C) density
D) number of molecules present
Correct Answer:

Verified
Correct Answer:
Verified
Q108: If the pressure in a gas container
Q109: Pressure is defined as<br>A)force divided by unit
Q110: Gases do not behave ideally under conditions
Q111: Assume that you have a sample of
Q112: The pressure in the eye of a
Q114: If the pressure in a gas container
Q115: According to Graham's law,the rate of effusion
Q116: Some assumptions from the kinetic molecular theory
Q117: In the laboratory,hydrogen gas is usually made
Q118: At 298 K which is larger rate