Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram. 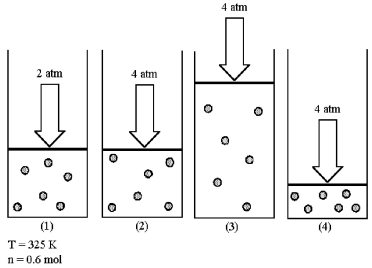
-Which diagram (2) -(4) most closely represents the result of doubling the pressure and doubling the temperature while keeping the number of moles of gas constant?
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q106: What is the Celsius temperature of 100.0
Q107: Effusion of a 1:1 mixture of two
Q108: If the pressure in a gas container
Q109: Pressure is defined as<br>A)force divided by unit
Q110: Gases do not behave ideally under conditions
Q112: The pressure in the eye of a
Q113: You are given two flasks of equal
Q114: If the pressure in a gas container
Q115: According to Graham's law,the rate of effusion
Q116: Some assumptions from the kinetic molecular theory