Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the pressure and number of moles of gas while keeping the temperature constant? 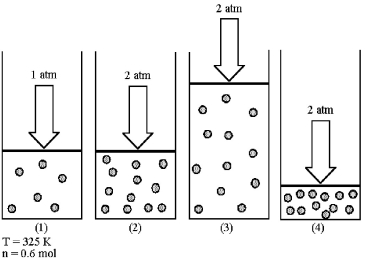
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q26: Some assumptions from the kinetic molecular theory
Q27: One mole of gas at 25°C has
Q28: The region of the atmosphere that is
Q29: Which of the following gases has the
Q30: How many liters of oxygen are needed
Q32: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q33: Which of the following is equivalent to
Q34: You have two samples of the same
Q35: A glass tube has one end in
Q36: Three identical flasks contain three different gases