Multiple Choice
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 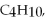 flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain same number of molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Assume that you have a sample of
Q32: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q33: Which of the following is equivalent to
Q34: You have two samples of the same
Q35: A glass tube has one end in
Q37: If the Earth's ozone (O<sub>3</sub>)layer has a
Q38: A basketball is inflated to a pressure
Q39: According to the kinetic molecular theory of
Q40: A gas occupies 22.4 L at STP
Q41: Two moles of neon gas at 20.0°C