Multiple Choice
The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 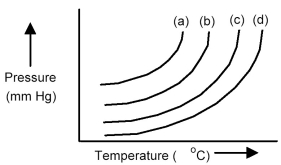
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for water?
A) curve (a)
B) curve (b)
C) curve (c)
D) curve (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q26: What phase changes occur when the temperature
Q27: From the plot of vapor pressure as
Q28: Aluminum has a face-centered cubic structure and
Q29: The coordination number of each atom in
Q30: While mercury is very useful in barometers,mercury
Q32: Which of the following compounds forms a
Q33: For which of the following phase changes
Q34: Which of the following forms a molecular
Q35: When a substance melts at its normal
Q36: The boiling point of a liquid is