Multiple Choice
From the plot of vapor pressure as a function of temperature shown below,the normal boiling point for tert-butyl alcohol is approximately 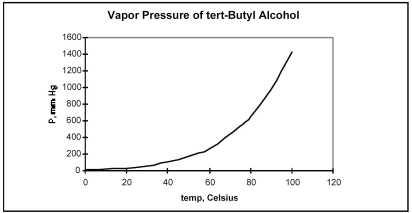
A) 0°C.
B) 40°C.
C) 85°C.
D) 100°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: The highest coordination number for spherical packing
Q23: When a narrow diameter glass tube is
Q24: Identify the packing in the figure shown
Q25: Hydroquinone is an antioxidant that is also
Q26: What phase changes occur when the temperature
Q28: Aluminum has a face-centered cubic structure and
Q29: The coordination number of each atom in
Q30: While mercury is very useful in barometers,mercury
Q31: The plots below represent vapor pressure vs.temperature
Q32: Which of the following compounds forms a