Multiple Choice
Drawing (1) shows a system in which an equilibrium exists between dissolved and undissolved gas particles at P = 1 atm.According to Henry's law,if the pressure is decreased to 0.5 atm and equilibrium is restored,which drawing (2) -(5) best represents the equilibrium at 0.5 atm? 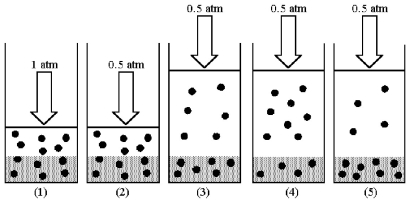
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q188: What is the weight percent of vitamin
Q189: A solution of 62.4 g of insulin
Q190: What is the freezing point of a
Q191: Assuming that sea water is a 3.5
Q192: The following diagram shows a close-up view
Q194: A solution is 4.50% by weight NaHCO<sub>3</sub>.How
Q195: Which of the following aqueous salt (NaCl)solutions
Q196: When 0.500 g of vitamin K is
Q197: Chloroform has a boiling point of 61.1°C
Q198: Aqueous solutions of 30.0% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are