Multiple Choice
Drawings (1) and (2) show the equilibrium vapor pressures of two pure liquids.Which drawing (3) -(6) represents the equilibrium vapor pressure of a solution made by mixing equal moles of each liquid? 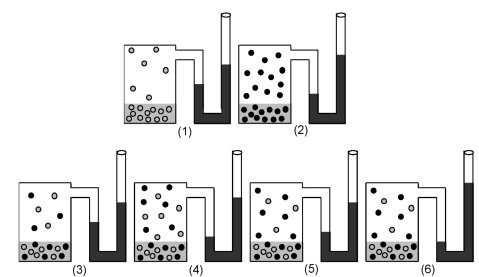
A) drawing (3)
B) drawing (4)
C) drawing (5)
D) drawing (6)
Correct Answer:

Verified
Correct Answer:
Verified
Q25: How will the osmotic pressure of an
Q26: A solution is prepared by dissolving 171
Q27: Which ion-dipole interaction results in the larger
Q29: How much water must be added to
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q32: A person is considered legally intoxicated with
Q33: Calculate the freezing point of a solution
Q34: Which of the following should most favor
Q35: A solution is prepared by dissolving 17.75
Q40: Arrows in the energy diagram below represent